Get help
Why use Findmyassay.com?
High quality biomedical research begins with adequate quality biospecimen samples. Before you start any experiment, you’ll want to verify the quality of your clinical samples and make sure they are suitable for their intended downstream purpose.
You are a researcher, a biobanker or you have legacy collection of biospecimens in your freezers. You would like to use them in a research project, but you are unsure of their quality. Instead of using them blindly, as “convenience samples”, you should use them with confidence (or …throw them away)!
Findmyassay.com is a free, simple and user-friendly tool that will help you identify relevant assays to qualify or annotate your clinical samples and increase their value to researchers.
Whether you are using fluid biospecimens, nucleic acids, cells or tissue, you can characterise your samples and perform quality control checks. Using samples that are fit for purpose, you can avoid wasting precious material and resources.
How does Findmyassay.com work?
Simply follow the steps to select the appropriate biospecimen type and sub-types to identify the relevant quality attributes and receive clear suggestions on which assays to use.
Don’t waste precious material. Ensure your samples are fit-for-purpose.
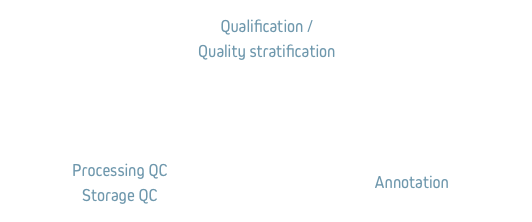
The proposed assays are either qualification or quality stratification assays. Some of the proposed assays correspond to processing or storage quality control assays (see definitions). Some of the proposed assays correspond to quantitative annotations that may be relevant in certain downstream sample uses.
Definitions
Characterisation: biospecimen annotations with analytical measurements that may define biospecimen’s fitness-for-purpose.
Processing Quality Control: measurements of analytes, known to be sensitive to in-vitro preanalytical variations. This quality control leads to qualification or qualitative stratification of the analysed biospecimens.
Storage Quality Control: measurements of analytes in the context of long-term biospecimen stability studies. This quality control may lead to qualification or qualitative stratification of the biospecimens.
Qualification: verification of biospecimen’s fitness-for-purpose for research use – either in a specific disease area or on a specific downstream analytical platform – based on objective analytical evidence.
Qualitative stratification: categorisation of biospecimens into two, three or more categories, – based on objective analytical evidence – , each category corresponding to a specific in-vivo biological characteristic (e.g. level of inflammation, % tumor) or to a specific ex-vivo pre-analytical condition (e.g. pre-centrifugation time). In the absence of scientific data on the preanalytical robustness of a biomarker, this categorisation data can be used in future biomarker analyses as significant co-variables.